CHEMISTRY | QN 01 | MOLE CONCEPT
What volume of water will be produced at room temperature and pressure when 2g of hydrogen is burnt in air?
-
Data:
Mass of hydrogen gas (H2) = 2g
Molar volume at room temperature and pressure = 24 dm3
Asked: volume of water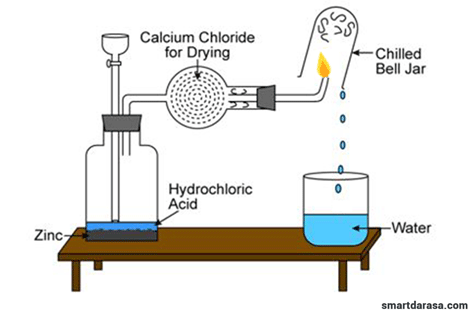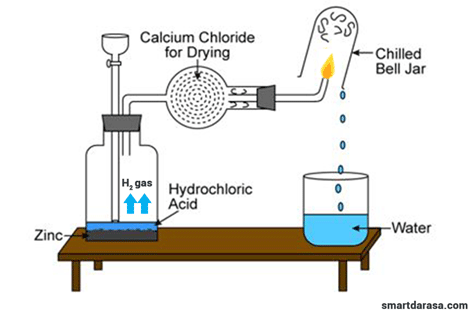
-
Chemical equation for the reaction.
2H2(g) + O2(g) → 2H2O(L)
Mole ratio 2:1:2 -
To convert 2g of H2 into moles then to relate
Mole = mass/Molar mass
= 2g / 2g/mole
= 1 mole of H2
Mole equivalent between H2 and water.
2moles of H2 Ξ 2 moles of H2O
1mole of H Ξ ?? of H2
= (1 moles × 2 moles) / 2 moles
= 1 mole of H2 -
To convert 1 mole of H2O into volume of water.
Mole = volume / Molar volume
Volume = mole × molar volume
= 1 mole × 24dm3/mole
= 24dm3
24dm3 of water was produced at S.T.P.

More questions
CHEMISTRY questions are much EASIER when you clear understand the concept. Smart Darasa provides space for teachers to contribute concepts and questions solutions in fun and interactive way.
Prepared By:
Sir Mahame
Biology & Chemistry teacher,
Mapambano Education Center | Mwenge | DSM.
Contacts: +255 757 609 664 | +255 655 619 664
Send Your CHEMISTRY Question
Are you a STUDENT | PARENT | TEACHER, Send your CHEMISTRY question for FREE and we will get back to you with the solution or relavant link with similar question. We receive questions every day from 08:00hrs to 16:00 EAT. Depend on volume of questions, we send solutions the same day from 18:00hrs to 20:00hrs EAT or later next day.